KOAG expands the patented Vascette® technologies
Vascette® HP Vascette® Max Vascette® PICC
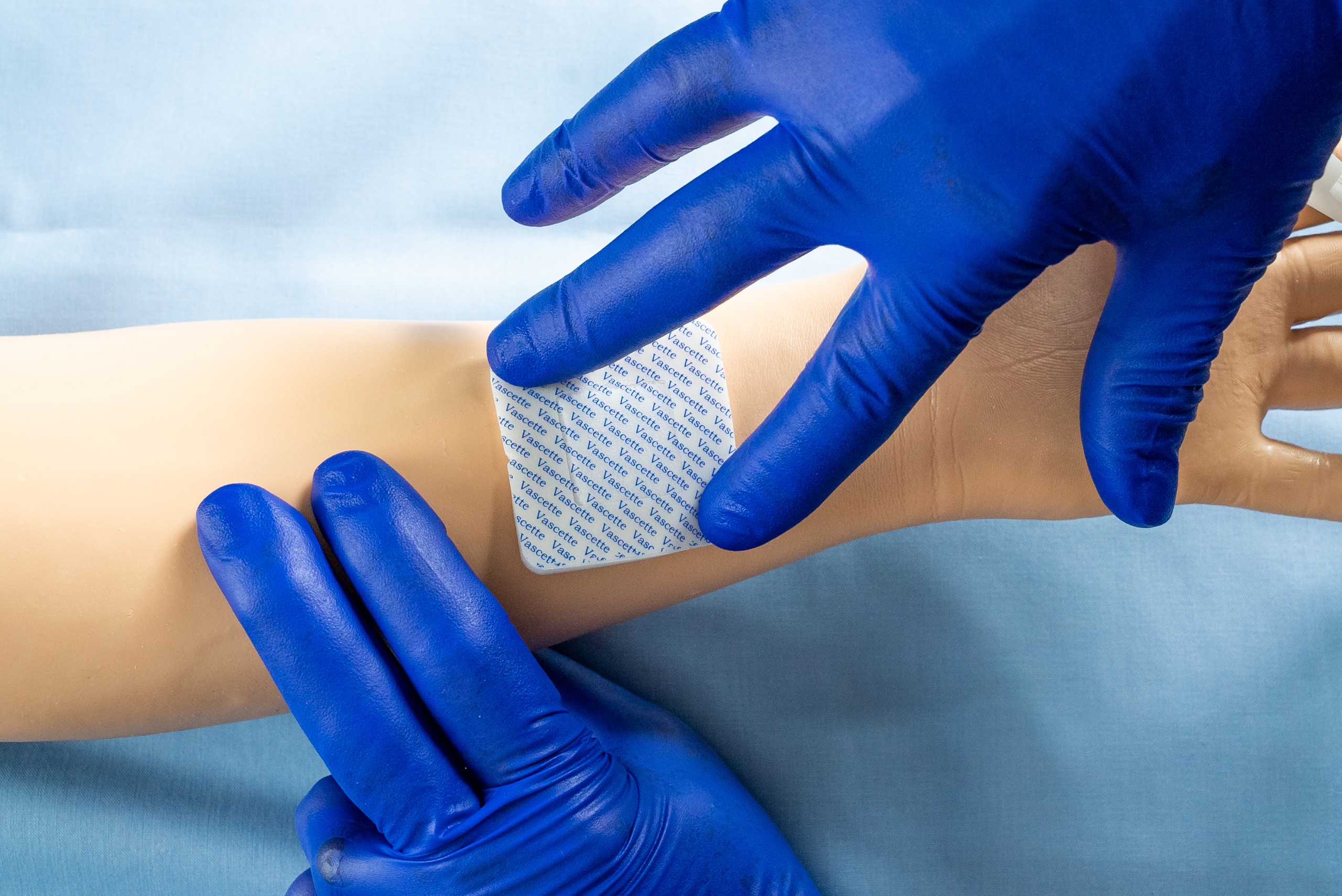
Vascette® advances the standard of care with a plant-based, no compression solution to successful vascular closures. With Vascette® there is no compression force over the arteriotomy site.
Simple Peel and Press Application
Faster Time to Hemostasis
No Arteriotomy Compression
Reduced Recovery Times
About Us
KOAG LLC was established to develop and globally distribute innovative topical devices for applications in vascular interventions. We have designed and tested the Vascette® plant-based technologies for both radial and femoral closures and PICC line management.
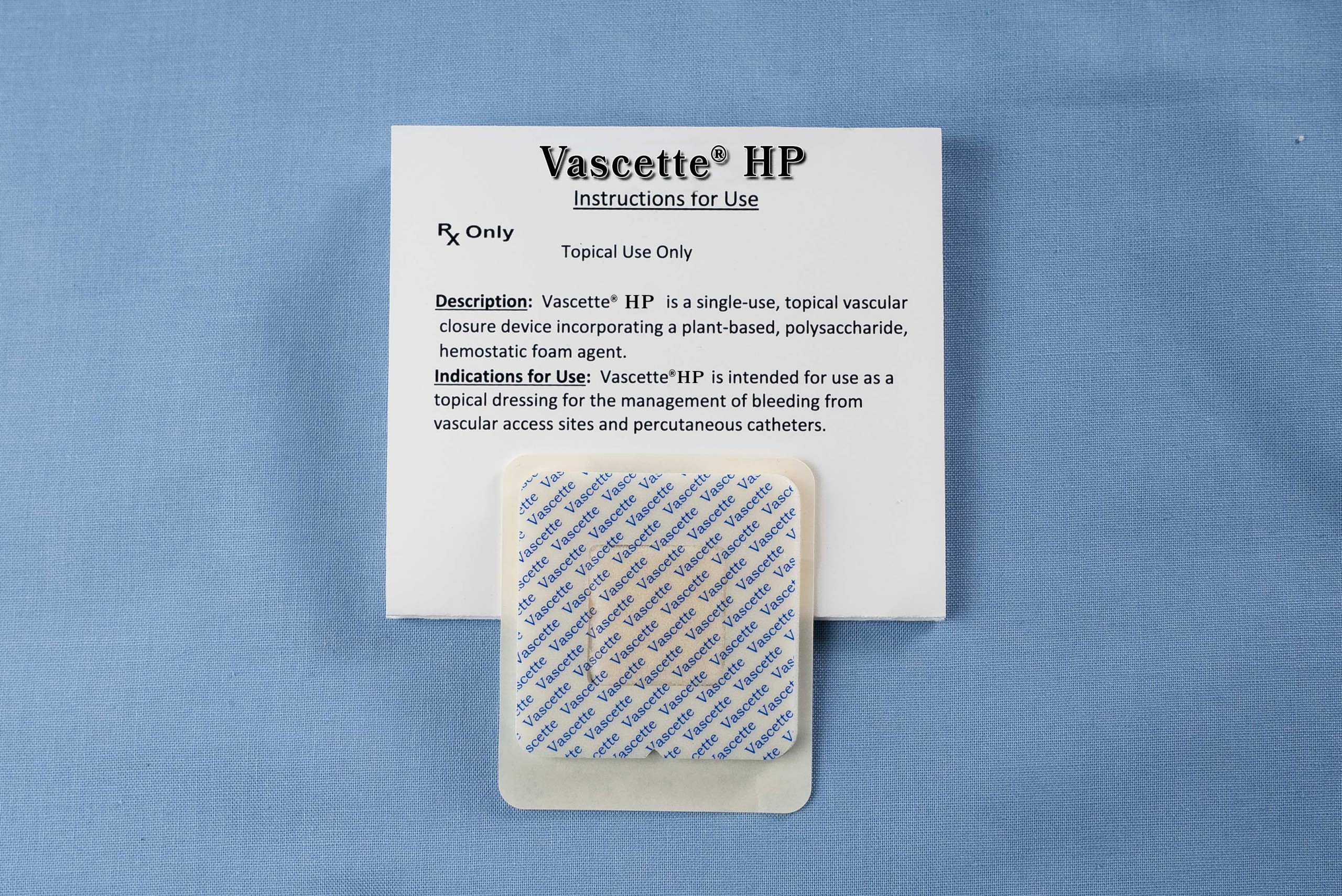
Vascette® is a patented, peel and press dressing incorporating a robust, surgical foam hemostat.
Vascette® HP is cleared by the U.S. FDA.
Our Team
David Lang
Chairman & CEO
Steve Muellerleile
Director & Vice President of Operations
Chuck Roman
Director U.S. Sales & Marketing
Kathy Simpson
Director of Regulatory Affairs
Donald Sturtevant
Director
Ted Costopoulos
Director
Peter Guenther
Director
July 30, 2022: KOAG announces FDA clearance of its Vascette® HP topical Hemostatic Pad for vascular closure.